Research Project
Tissue homeostasis is highly regulated where the extracellular matrix plays multiple and essential roles. If this control is lost severe life threatening diseases can occur such as chronic inflammation, myocarditis, kidney failure and cancer. Understanding the molecular mechanisms of matrix actions will allow to fight cancer and other pathologies.
More:
Tumors represent multi-cellular ecosystems where transformed tumorigenic and stromal cells talk to each other and respond to their surrounding specific extracellular matrix that they produce. The extracellular matrix of this tumor bed does not only serve as architectual scaffold but also as reservoir for soluble factors providing mechanical and biochemical information, altogether described by the term « tumor microenvironment ». The tumor microenvironment largely impacts cell behaviour but how is poorly understood. There is ample evidence for the extracellular matrix molecule tenascin-C to regulate tissue maintenance as well as to promote disease malignancy when excessively expressed.
The underlying molecular mechanisms how tenascin-C exerts pleiotrophic effects in cancer was difficult to assess due to the lack of relevant in vivo models that appropriately mimic the complexity of cancer tissue. To respond to this challenge, the team has developed several stochastic and autochthonous murine tumor models as well as ex vivo and in vitro models with abundant and no tenascin-C. In particular, the laboratory had developed the first transgenic tumor model with combined no and high tenascin-C expression that formally demonstrated a pivotal role of tenascin-C in tumor progression (Saupe et al., 2013, Cell Reports).
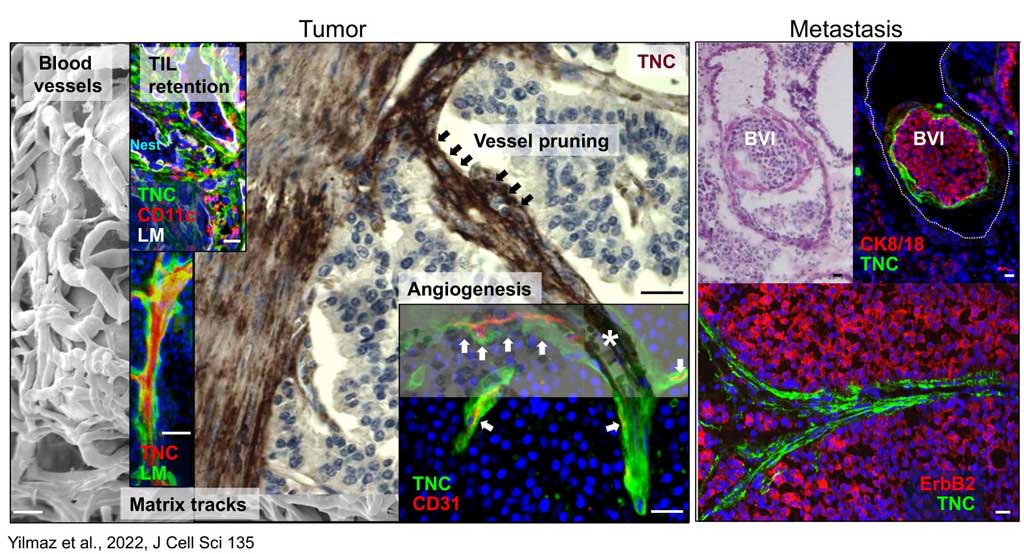
Using state of the art biochemical and cell biological assays, genomics and proteomics as well as tissue imaging the lab has generated significant mechanistic and molecular insights into the structure – function roles of tenascin-C in cancer progression. The laboratory demonstrated that tenascin-C promotes the angiogenic switch and metastasis. The laboratory further showed that tenascin-C forms fibrillar matrix tracks inside the tumor stroma, serving as niches for tumor and stromal cells.
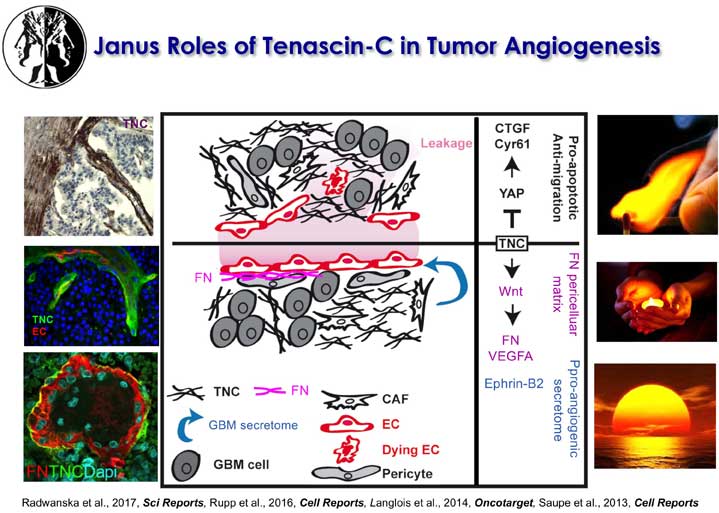
The laboratory further showed that tenascin-C plays a Janus role in tumor angiogenesis with distinct mechanisms promoting and inhibiting blood vessel formation, respectively. Many of these results have prognostic value in cancer diagnosis as e.g. the observation that low tenascin-C levels (which highly exceed tenascin-C levels in normal tissue) is already dangerous because tenascin-C triggers migration and metastasis through a tumor cell intrinsic mechanism (Sun et al., 2018, Cancer Res). Using the novel immune competent tumor models with regulated tenascin-C expression furthermore allowed the team to reveal important roles of tenascin-C in orchestrating tumor immunity defining the novel terms of Tumor Matrix Tracks (TMT) and TIL-Matrix-Retention (Spenle et al., 2020, Cancer Immun Res) and revealed that tenascin-C is promoting several hallmarks of cancer (reviewed in Yilmaz et al., 2022, J Cell Sci). A major goal of the teams is the transfer of knowledge to improve prediction and to develop novel anti-cancer treatment protocols.
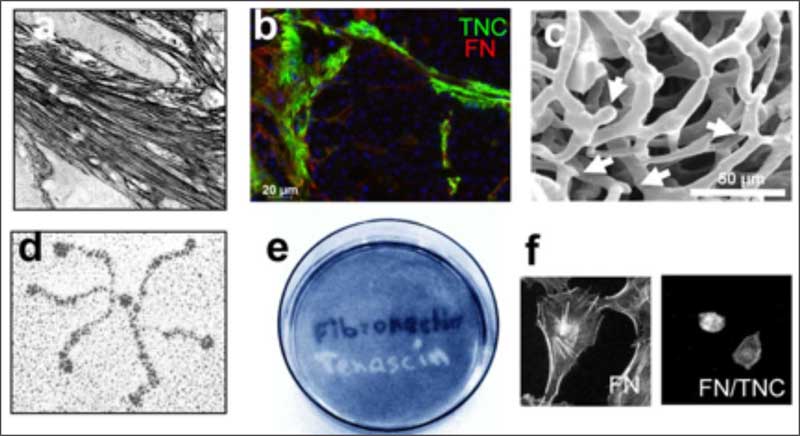
The lab has discovered the MAtrix REgulating MOtif that mediates the interaction of tenascin-C with fibronectin, another matrix molecule (Loustau et al., 2022, Mat Bio). Synthetic peptides mimicking this motif abolish many of the tenascin-C enforced cancer hallmarks and importantly, restores anti-tumor immunity thus reducing tumor growth (Li et al, 2024, PNAS). These peptides also act as “matrix-gate-openers”. Their application in combination with other anti-cancer therapies where the dense fibrotic matrix is an obstacle may revolutionize cancer therapy.
Research objectives of the laboratory:
- Establish murine models recapitulating human tumor matrix and immunity parameters
- Determine how tenascin-C regulates tumor immunity and events on the road to metastasis
- Develop prognostic parameters for cancer patient survival and selection of therapy
- Restore tumor immune surveillance by targeting tenascin-C
- Develop novel tenascin-C targeting tools
To better understand the roles of tenascin-C murine models with engineered tenascin-C levels were generated:
PNET insulinoma to study angiogenesis (Saupe et al., 2013, Cell Rep 5; Langlois et al., 2014, Oncotarget 5)
MMTV-NeuNT breast cancer to study immunity and metastasis (Sun et al., 2019, Mat Bio 83; Deligne et al., 2020, Cancer Immun Res 8; Spenle et al., 2020, Cancer Immun Res 8; Murdamoothoo et al., 2021, EMBO Mol Med 13)
Autochthonous NT193 breast cancer to study immune editing, TMT formation and TIL-Matrix-Retention (Sun et al., 2019, Mat Bio 83; Deligne et al., 2020, Cancer Immun Res 8; Murdamoothoo et al., 2021, EMBO Mol Med 13)
Carinogen-induced tongue oral squamous cell carcinoma (OSCC) to study matrix immune suppression (Spenle 2021, Cancer Immun Res 8)
Radiosensitive syngeneic tongue OSCC13 grafting to study matrix regulation of irradiation-induced tumor remission (Spenle et al., 2021, Front Immun 12)
AAV kidney disease (Anti neutrophil cytoplasmic antibody associated vasculitis) to study matrix regulation of kidney necrosis/sclerosis (Abou-Faycal et al., 2022, Mat Bio 106)
Major Results:
The laboratory showed that tenascin-C plays a Janus role in tumor angiogenesis by employing distinct molecular mechanisms thus promoting the formation of new blood vessels that are however leaky (Saupe et al., 2013, Cell Rep 5). The results have prognostic value in cancer and showed that already tenascin-C levels below the median (however highly exceeding tenascin-C levels in normal tissues) correlates with shorter patient survival as tenascin-C triggers cancer cell migration and metastasis by autocrine and paracrine mechanisms (Sun et al., 2018, Cancer Res 78; Sun et al., 2019, Mat Bio 83). The laboratory demonstrated that tenascin-C promotes the angiogenic switch as one of the AngioMatrix molecules that defines this switch (Langlois et al., 2014, Oncotarget 5; Rupp et al., 2016, Cell Rep 17; Radwanska et al., 2017, Sci Rep 7). The laboratory further showed that tenascin-C forms fibrillar tumor matrix tracks (TMT) together with fibronectin, laminin and other matrix molecules inside the tumor stroma, thereby generating biochemical niches for tumor, stromal and immune cells (Spenle et al., 2015, Cell Adh Migr 9). Using the novel immune competent tumor models with engineered tenascin-C expression furthermore allowed the team to discover so far unknown actions of tenascin-C in orchestrating tumor immunity. Tenascin-C induces and binds several chemokines thus generating a sticky substratum that retains tumor infiltrating leukocytes (TIL) in the stroma, representing the immune exclusion phenotype, thereby blocking TIL contact with the tumor cells. This TIL-Matrix-Retention mechanism can be targeted by inhibition of specific tenascin-C actions (Spenle et al., 2020, Cancer Immun Res 8; Deligne et al., 2020, Cancer Immun Res 8; Murdamoothoo et al., 2021, EMBO Mol Med 13) in particular with novel tenascin-C specific nanobodies (Dhaouadi et al., 2021, Front Immun 12) and the MAREMO peptides (Loustau et al., 2022, Mat Bio 108, Li et al., 2024, PNAS), altogether substantiating the concept of targeting matrix to improving immune checkpoint and anti-cancer therapy.